Background:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is theoretically the only curative option for high-risk acute myeloid leukemia (AML) patients. However, many patients relapse after allo-HSCT, and treatment options are very limited. Azacitidine, a DNA methyltransferase inhibitor exhibits anti-leukemia activity in myeloid diseases. Chidamide, a selective histone deacetylase inhibitor, effectively induces apoptosis of AML cells. They have synergistic inhibitory effect on AML cells when combined together. Therefore, we conducted a multicentre, prospective study (ChiCTR2300067593) to examine the tolerability and efficacy of a combination of both chidamide and azacitidine as a maintenance therapy for high-risk AML patients after allo-HSCT.
Patients and methods:
Patients ≤ 60 years of age with high-risk AML post- allo-HSCT were enrolled. The high-risk characteristics of AML were defined as one or more of the following criteria: a) Patients with poor prognosis according to the genetic risk stratification assessment of European Leukmia Net (ELN) in 2022; b) Primary refractory or recurrent AML; c) Secondary AML (secondary to MDS or treatment-related AML). All patients received Chidamide 5 mg/d combined with azacitidine 75 mg/m2/d for 5 days. The cycle interval was 6-8 weeks. A total of 6 cycles was recommended. Treatment started as early as 3 months after transplantation. The primary endpoint of this study was cumulative incidence of relapse (CIR). The secondary endpoints included rates of overall survival (OS) and event-free survival (EFS). Survival outcomes were estimated using Kaplan-Meier analysis.
Results:
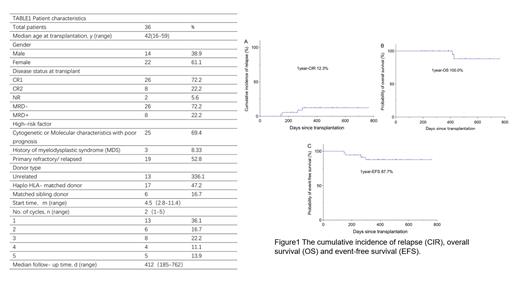
Thirty-six patients have been recruited. The median age was 42 years (range, 16-59). Baseline characteristics are shown in Table 1. With a median follow-up time of 412(185-762)days, the 1-year CIR was 12.3 (6.5-18.1) %, the 1-year OS and EFS rates were 100.0% and 87.7 (81.9-93.5) %, respectively. The median OS and EFS time were not reached. Four patients relapsed, two died, and two are still alive and ready for the second transplantation. The most common adverse events (AEs) were thrombocytopenia reaction and gastrointestinal tract reaction. Grade 2 or 3 AEs were observed in 16.7% (6/36) and 25.0% (9/36) of the patients, respectively. No grade >3 AEs were noted. 9 patients (6/36,16.7%) developed grade 3-4 thrombocytopenia, without active hemorrhage. All of them received blood transfusion and thrombopoietin receptor agonist. The median persistence of thrombocytopenia was 3 (2-5) d. Chronic graft-versus-host-disease (cGVHD) occurred in 25.0% (9/36) of patients. Four cases (4/36, 11.1%) were complicated with infection. They were one case of intestinal infection, one case of pulmonary and EBV infections, one case of hepatitis B virus activation and one case of herpes zoster. And all infected patients recovered safely after anti-infection and symptom-oriented treatment. CMV activation was not observed. Non relapse mortality (NRM) was zero.
Conclusions:
We conclude that chidamide plus azacitidine can be administered safely after allo- HSCT with no evidence of an increased incidence of GVHD, and this combination may decrease the relapse rate in high-risk AML patients. This novel maintenance therapy may be a promising way to prevent relapse in high-risk AML patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal